Information Release
Wednesday, December 28, 2022
A clinical demo led by the Countrywide Cancer Institute (NCI), part of the National Institutes of Health and fitness, has resulted in the very first approval of a cure for highly developed alveolar gentle part sarcoma (ASPS). The immunotherapy drug atezolizumab (Tecentriq) was not long ago permitted by the U.S. Food items and Drug Administration (Food and drug administration) for the treatment of adults and small children 2 decades and more mature with ASPS that has distribute to other sections of the human body or simply cannot be taken out by surgical procedure.
ASPS is an exceptionally rare most cancers that influences mainly adolescents and youthful grown ups. The acceptance was primarily based on info from a non-randomized phase 2 demo (NCT03141684) funded by NCI and led by Dr. Alice Chen, M.D., of the Developmental Therapeutics Clinic in NCI’s Division of Cancer Therapy and Diagnosis (DCTD). Genentech, a member of the Roche Team and the company of atezolizumab, presented the drug to NCI through a cooperative research and growth settlement. The results of the study are currently being organized for publication.
“Forty per cent of the individuals ended up handled at the NIH Clinical Centre in Bethesda,” claimed James H. Doroshow, M.D., director of DCTD. “Our means to provide sufferers in from all over the planet was a important element in the capability to do the research.”
“This acceptance will make a big effects in phrases of a rare condition that has been particularly difficult to handle,” Dr. Chen mentioned.
This is the largest review on ASPS. It is also the initially examine performed in the NCI-funded Experimental Therapeutics Medical Trials Community that has resulted in a drug acceptance. The network enabled sarcoma experts at academic professional medical centers from across North The usa to enroll individuals in the trial.
“This is a big milestone for investigators in the Experimental Therapeutics Scientific Trials Community, as perfectly as for the ASPS client neighborhood, and for analysis on uncommon cancers,” mentioned Elad Sharon, M.D., of DCTD, who is one particular of the study leaders.
This is also the first time atezolizumab has been authorized for young children. Dr. Chen pointed out that this was enabled by the participation of the Pediatric Oncology Branch in NCI’s Middle for Most cancers Study, which helped enroll small children in the trial.
“This research is an important illustration of collaboration amongst pediatric and health-related oncology, allowing little ones with quite uncommon cancers accessibility to powerful new therapies,” explained John W. Glod, M.D., Ph.D., of the Pediatric Oncology Branch. “The total review crew is grateful to the people who participated in the review and made this get the job done possible.”
About 80 persons in the United States are identified with ASPS each individual calendar year. The ailment generally starts in the gentle tissue that connects and surrounds the organs and other tissues. Although the illness grows slowly, at the time it spreads it is generally fatal, and chemotherapy is ineffective. About 50{33c86113bcc32821f63c6372852a0f501e07fff55ce3ce61b15b246c5f8c531c} of individuals with metastatic illness are nevertheless alive following five several years. New specific treatments, together with prescription drugs known as tyrosine kinase inhibitors, do not have lasting efficiency. Not long ago, however, immunotherapy prescription drugs have shown guarantee as attainable therapies for ASPS.
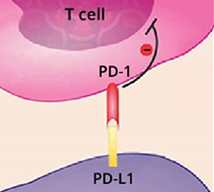
Atezolizumab is an anti-PD-L1 immune checkpoint inhibitor that performs by helping the immune system answer a lot more strongly to most cancers. Food and drug administration has accredited atezolizumab for the therapy of sufferers with quite a few cancer sorts, which includes liver cancer, melanoma, and lung most cancers.
In 2020, Food and drug administration granted breakthrough therapy designation for atezolizumab to take care of clients with unresectable or metastatic ASPS. This designation usually means that atezolizumab, which is intended to deal with a serious affliction, had fulfilled FDA’s criteria for expedited development and evaluation of the drug. Afterwards that 12 months, Food and drug administration granted orphan drug designation to atezolizumab for comfortable tissue sarcoma in typical. This status presents incentives for businesses to acquire a drug for unusual conditions.
The phase 2 trial enrolled 49 ethnically numerous sufferers ages 2 and older with metastatic ASPS, who have been offered an infusion of atezolizumab every 21 days. About a 3rd of the people responded to the remedy with some degree of tumor shrinkage, according to their doctor’s assessment. Most of the other individuals expert steady ailment.
Soon after two years of procedure, sufferers have been specified the chance to quit treatment method and go on a treatment split for up to two several years with near monitoring. None of the individuals who took a therapy break had disorder development through that time.
Critical aspect effects happened in 41{33c86113bcc32821f63c6372852a0f501e07fff55ce3ce61b15b246c5f8c531c} of individuals receiving atezolizumab these incorporated anemia, diarrhea, rash, dizziness, hyperglycemia, and suffering in the extremities. However, no people arrived off the review for the reason that of facet effects.
“This approval signifies a victory for exceptional disorders, which are understudied in clinical trials,” said Dr. Chen. “For this approval to go via in a unusual disease, and to be able to make an impression on these younger people’s life, is pretty sizeable.”
Analysis teams are now conducting further trials with atezolizumab for sufferers with ASPS, like giving the drug in mix with other therapies.
About the Countrywide Cancer Institute (NCI): NCI leads the National Most cancers System and NIH’s attempts to significantly cut down the prevalence of most cancers and improve the life of cancer people and their families, by means of investigation into avoidance and cancer biology, the development of new interventions, and the teaching and mentoring of new researchers. For a lot more information and facts about cancer, remember to check out https://www.most cancers.gov or get in touch with NCI’s speak to heart, the Cancer Details Provider, at 1-800-4-Most cancers (1-800-422-6237).
About the Nationwide Institutes of Wellbeing (NIH):
NIH, the nation’s healthcare research agency, includes 27 Institutes and Facilities and is a part of the U.S. Office of Health and Human Services. NIH is the major federal company conducting and supporting basic, clinical, and translational healthcare study, and is investigating the causes, treatment options, and cures for the two typical and scarce conditions. For far more facts about NIH and its packages, check out www.nih.gov.
NIH…Turning Discovery Into Overall health®




More Stories
White House plans to nominate cancer center chief to lead NIH
What Happens to the Brain in Alzheimer’s Disease?
Biden Plans to Pick Monica Bertagnolli to Lead National Institutes of Health