This posting is the sixth in our “Developments in digital wellbeing” sequence.
Any person who has used extra than a passing moment browsing a smartphone application retail outlet will know that overall health applications have by no means been additional widespread. All varieties of software which could be explained as a “health app” can be found on your app retail store of preference.
A lot of overall health applications are offered completely lawfully. However, numerous are not.
The reason for this is that some health and fitness apps cross a critical regulatory line. As an alternative of stopping at staying mere buyer applications which do wellness-relevant issues, they are health-related gadgets, and so are issue to a host of regulatory demands.
What will make a healthcare product?
Broadly speaking, United kingdom and EU professional medical product polices define a “medical device” as a solution (including standalone software) which does not obtain its principal intended motion by pharmacological, immunological or metabolic indicates and which is intended by its maker to be used for just one of a shut checklist of healthcare applications:
- prognosis, avoidance, monitoring, prediction, prognosis, treatment or alleviation of condition
- diagnosis, monitoring, procedure, alleviation of, or compensation for, an damage or incapacity
- investigation, substitution or modification of the anatomy or of a physiological or pathological course of action or condition
- delivering information and facts by means of in vitro assessment of specimens derived from the human human body, which include organ, blood and tissue donations.
Certain sorts of product, including solutions to control or support conception, are also deemed to be professional medical products even although they do not have a health care objective.
A product does not have a health care goal if it is not meant to be applied for the profit of an individual individual.[1] Counterintuitively, this implies that a contact tracing application which estimates the prevalence of a disorder in a community for general public wellness reasons is not likely to be a medical machine since it does not have a health care goal. In the meantime, an application which estimates the likelihood that an individual is struggling from a disease based on their reported signs or symptoms is probable to be a clinical gadget since it has a health-related function, particularly prognosis of illness.
The intended use of a device is judged objectively, based mostly on the presentation of the unit in its directions for use and its promotion supplies and the obvious performance of the system. As a end result:
- A health app can be a healthcare unit mainly because of the claims which the producer would make about the wellbeing application. For instance, if a manufacturer statements that its wellbeing app can diagnose Parkinson’s disease, the health and fitness app will most likely be a health care gadget even if it does not have this features.
- Disclaimers these types of as “this app really should not be utilised to diagnose Parkinson’s disease” are usually ineffective. Disclaimers can often make clear the intended use of the wellbeing app, but not if the disclaimer is clearly inconsistent with the broader presentation of the well being application.
In other phrases, a overall health app will be a clinical product where an aim third celebration would consider that the wellness application is intended to be used for a professional medical goal for the benefit of an person affected person, based on its presentation and clear features.
Unsurprisingly, it can need specific assessment to determine regardless of whether or not a overall health app is or is not a clinical device in the Uk and in the EU. Thanks to slight discrepancies in regulation and advice, there can be cases the place a individual application is deemed a healthcare device in the EU but not in the British isles, and vice versa.
Having said that, there are specified groups of wellbeing application which have a tendency to be clinical equipment, and sure types which are inclined not to be professional medical devices:
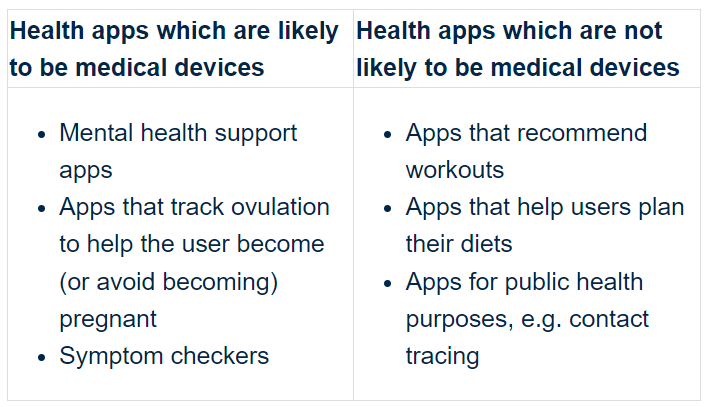
There are also particular phrases and phrases which have a tendency to reveal that a overall health app is a medical product, this kind of as: triage, places, detects, measures, finds, prognosis, predicts, monitor, symptom checker, threat of or lessen medical professional time.
What requirements does a software package medical unit have to comply with?
Health care products are subject to rigid regulatory necessities in the EU and in the Uk. 1 these necessity is that a medical system must endure a system to show compliance with applicable prerequisites (regarded as a conformity evaluation) and be labelled with a marking (both a CE Mark or a UKCA Mark) indicating that it complies with health-related gadget regulations right before it is placed on the market.
The goal of the conformity assessment is to validate that the health care unit is protected and that it performs in the fashion supposed by the company. Where by a health care system is deemed to existing a low chance, the producer can conduct the conformity evaluation itself and then self-certify that the health-related system is compliant. All other health care devices must be conformity assessed by an impartial 3rd bash (acknowledged as a Notified Human body).[2] In follow, almost all software clinical gadgets must go through conformity evaluation by a Notified Overall body.
Many health and fitness apps which are offered on application stores surface to have been effectively labelled with a conformity marking and so feel to have undergone conformity evaluation. Nevertheless, with out naming any names, there are a great deal of other individuals which do not bear a conformity marking, in spite of obviously acquiring an meant medical intent. There are also other wellness applications which have been labelled with a conformity marking, but which have incorrectly been self-certified as reduced chance by the producer, when they must have undergone conformity assessment by a Notified Human body.





More Stories
Durango church offers ambulatory medical equipment for lending – The Durango Herald
How Robotic Applications are Changing Medical Devices
Online tool analyzes at-home videos to predict musculoskeletal health